Strong Electrolyte And Weak Electrolyte
Electrolytes are substances that get ions when they become dissolved in h2o.
INTRODUCTION:
Where do we utilise electrolytes in daily life? Accept a guess. Don't we use common salt in our food? That's an electrolyte. Those special sports drinks offered to athletes are also electrolytes. The detergent we employ for washing our clothes is once again an electrolyte. These are a few instances wherein electrolytes play an important role in our solar day-to-twenty-four hour period lives.
 Source
Source
But THE QUESTION ARISES: WHAT IS AN ELECTROLYTE?
Electrolytes are substances that get ions (an atom or molecule with a net electrical charge) when they become dissolved in water and acquire the capacity to comport electricity. A positively charged ion is called a cation, whereas a negatively charged ion is called an anion.
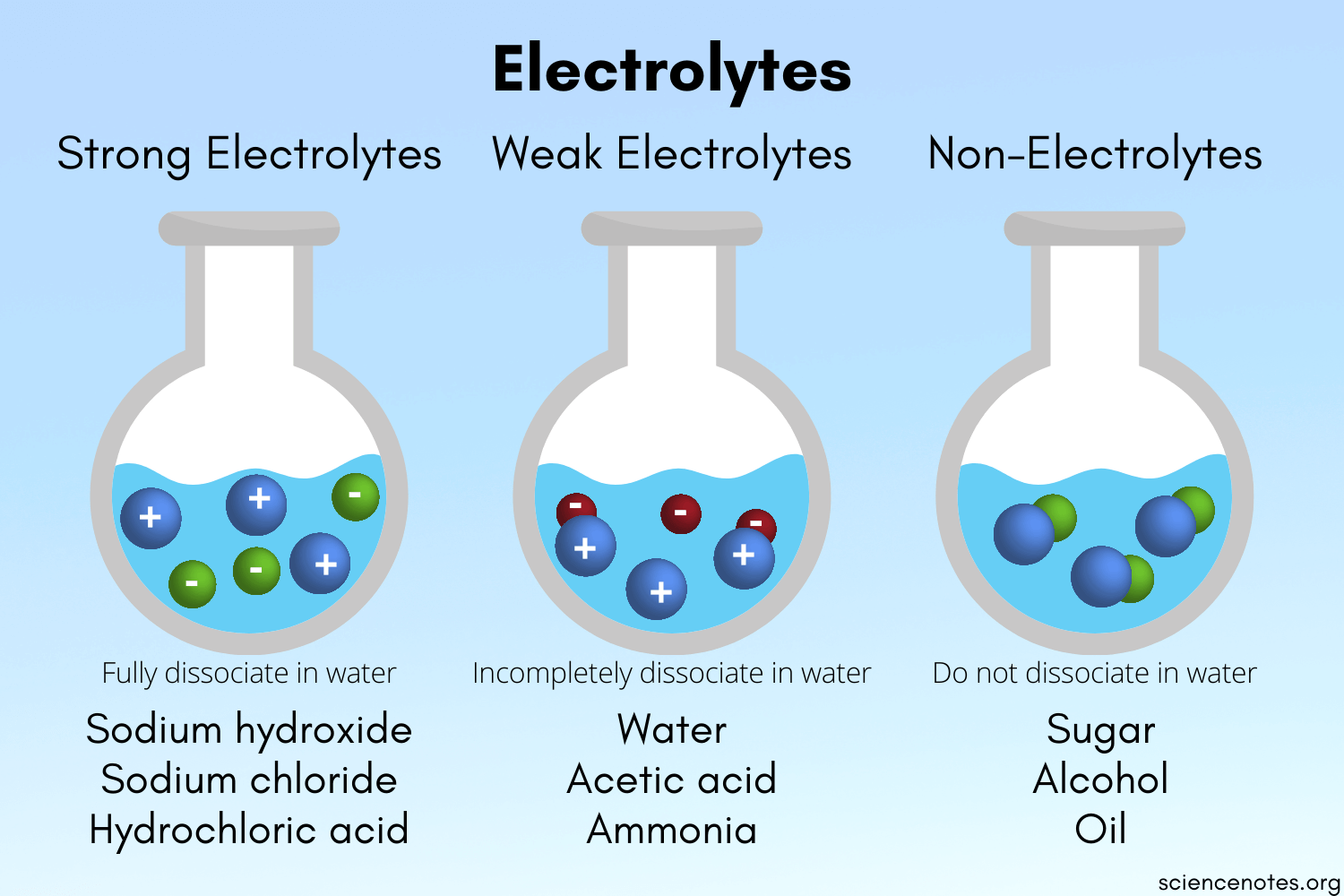
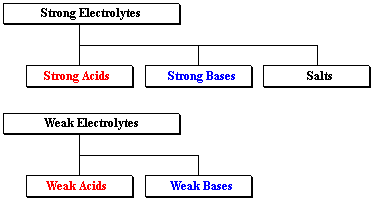
Based on ionization (dissociation into constituent ions) in an aqueous solution, electrolytes are of two types: strong and weak.
 Source
Source
STRONG ELECTROLYTE:
Potent acids, bases, and salts are examples of strong electrolytes. In an aqueous solution, these compounds dissociate into ions.
EXAMPLES:
- HCl - Hydrochloric acid
- HBr - Hydrobromic acrid
- Hello - Hydroiodic acid
- NaOH - Sodium hydroxide
- NaCl - Sodium chloride
WEAK ELECTROLYTE:
In water, weak electrolytes just partially disintegrate into ions. Weak acids, bases, and a range of other substances are examples of weak electrolytes. The majority of nitrogen-containing substances are weak electrolytes.
EXAMPLES:
- HF - Hydrofluoric acid
- CH₃CO₂H - Acerb acid
- NH₃ - Ammonia
- H₂O - Water
 Source
Source
Determination:
- An electrolyte is a substance that dissolves in water and acquires the capacity to behave electricity.
- There are ii types of electrolytes- strong and weak electrolytes.
- A strong electrolyte is an electrolyte that dissolves almost completely in water. An example of a potent electrolyte is Hydrogen Chloride (HCl).
- A weak electrolyte is an electrolyte that doesn't dissolve completely in water.
FAQs:
1. Is HCl acid a strong electrolyte?
Yes, hydrogen chloride is a strong electrolyte, and information technology dissolves in water completely to form Hydrogen ions and chlorine ions.
2. Why is HCl an electrolyte?
HCl is an electrolyte because it completely dissolves to form hydrogen ions and chloride ions when mixed with water. These together conduct electricity. Hence, it's an electrolyte.
3. When does HCl human activity equally a not-electrolyte?
In its pure form, HCl gas is a not-electrolyte. Electrolytes are formed one time it's dissolved in h2o. Since hydrogen chloride gas is not dissolved in h2o, information technology's a non-electrolyte.
Nosotros promise y'all enjoyed studying this lesson and learned something cool about Potent and Weak Electrolytes! Bring together our Discord community to get whatever questions you may have answered and to engage with other students just similar you! Don't forget to download our App to experience our fun, VR classrooms - nosotros hope, information technology makes studying much more fun! 😎
SOURCES:
- Strong and Weak Electrolytes. https://www.ck12.org/c/chemistry/strong-and-weak-electrolytes/lesson/Strong-and-Weak-Electrolytes-CHEM/ accessed 17 Feb 2022
- Chemistry Examples: Strong and Weak Electrolytes. https://www.thoughtco.com/potent-and-weak-electrolytes-609437 accessed 17 Feb 2022
Strong Electrolyte And Weak Electrolyte,
Source: https://www.inspiritvr.com/general-chemistry/water/strong-and-weak-electrolytes-study-guide#:~:text=A%20strong%20electrolyte%20is%20an,t%20dissolve%20completely%20in%20water.
Posted by: elkinsgoinfory.blogspot.com


0 Response to "Strong Electrolyte And Weak Electrolyte"
Post a Comment